517x
Filetype XLSX
File size 0.88 MB
Source: www.ema.europa.eu
File: Excel Sheet Download 11585 | Clinical Trial Information System Ctis Structured Data Form Initial Application Additional Member En | Sample Application
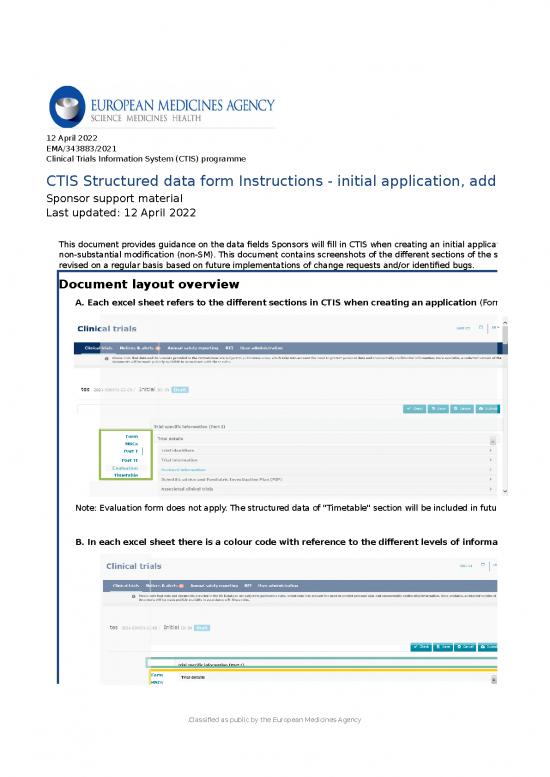
sheet 1 overview 12 april 2022 ema3438832021 clinical trials information system ctis programme ctis structured data form instructions initial application additional msc substantial and nonsubstantial modifications sponsor support material last ...
![icon picture XLSX icon picture XLSX]() Filetype Excel XLSX | Posted on 05 Jul 2022 | 3 years ago
Filetype Excel XLSX | Posted on 05 Jul 2022 | 3 years ago