304x
Filetype DOC
File size 0.08 MB
Source: assets.publishing.service.gov.uk
File: Certificate Word Format 30086 | Certiifcate Of Pharmaceutical Product Unlicensed Products Guidance Notes To Complete Application Form
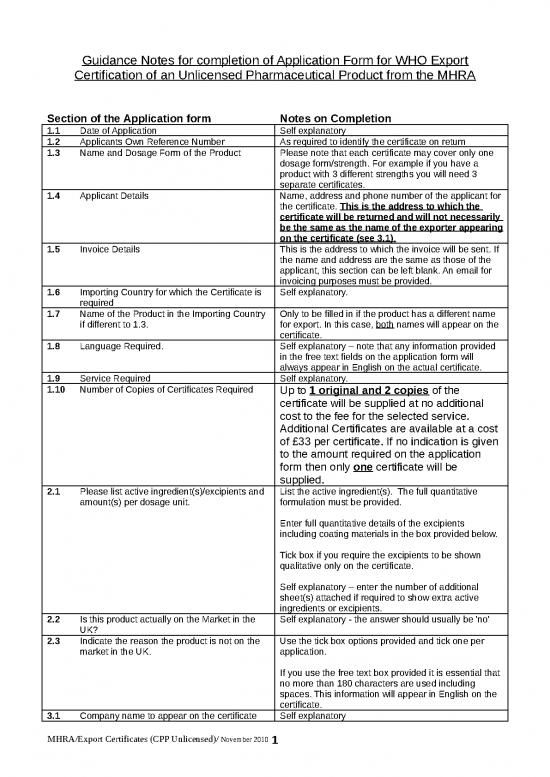
guidance notes for completion of application form for who export certification of an unlicensed pharmaceutical product from the mhra section of the application form notes on completion 1 1 date ...
![icon picture DOC icon picture DOC]() Filetype Word DOC | Posted on 07 Aug 2022 | 3 years ago
Filetype Word DOC | Posted on 07 Aug 2022 | 3 years ago