370x
Filetype PDF
File size 1.50 MB
Source: www.csus.edu
File: Day 2 Lecture
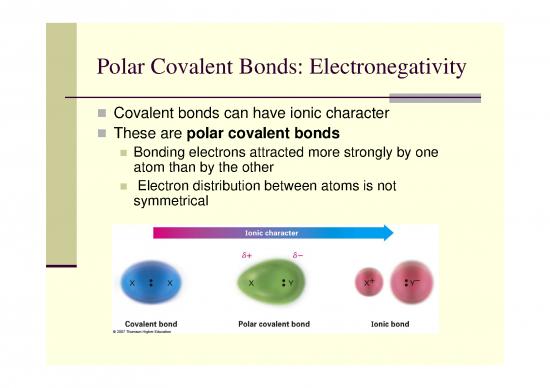
polarpolar ccovalentovalent bonds bonds electronegativityelectronegativity covalent bonds can have ionic character these are polar covalent bonds bonding electrons attracted more strongly by one atomatom thanthan byby thethe ootherther electron distribution ...
![icon picture PDF icon picture PDF]() Filetype PDF | Posted on 07 Feb 2023 | 3 years ago
Filetype PDF | Posted on 07 Feb 2023 | 3 years ago